Relacorilant for Cushing’s disease
What is relacorilant for Cushing’s disease?
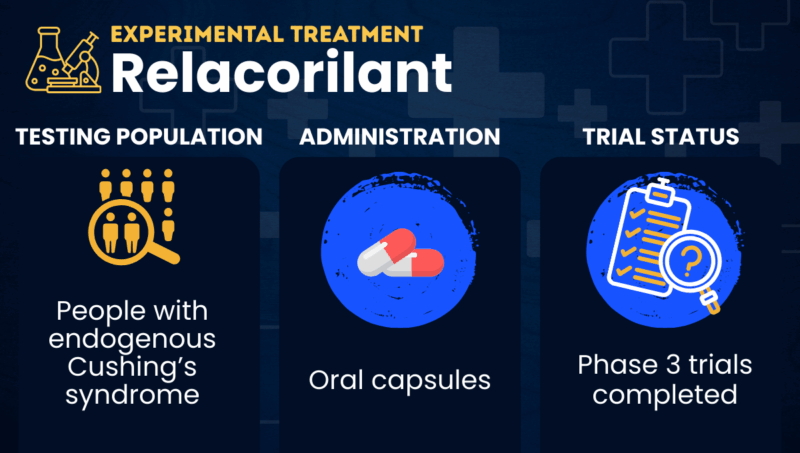
Relacorilant (previously known as CORT125134) is an oral therapy being developed by Corcept Therapeutics to treat people with endogenous Cushing’s syndrome, including Cushing’s disease.
In endogenous Cushing’s syndrome, problems within the body lead to the excess production of the hormone cortisol, driving symptoms such as high blood pressure (hypertension) and elevated blood sugar (hyperglycemia). Cushing’s disease is a form of the condition caused by a tumor in the brain’s pituitary gland.
Relacorilant aims to block the harmful consequences of excess cortisol by preventing the hormone from binding to glucocorticoid receptors, the proteins through which it normally exerts its biological effects.
In late 2024, Corcept sought approval of relacorilant in the U.S. for the treatment of Cushing’s patients with hypertension, with the medication to be administered in oral capsule form. The U.S. Food and Drug Administration rejected that application in late 2025, however, asking for additional evidence that the therapy’s benefits outweigh its risks. Corcept has indicated plans to work with the agency on a path toward regulatory approval.
Relacorilant holds orphan drug status in the U.S. and Europe for endogenous Cushing’s — a designation intended to help accelerate its development. Corcept is also testing relacorilant as a therapy for certain types of cancer.
Therapy snapshot
| Treatment name | Relacorilant |
| Administration | Oral capsules |
| Clinical testing | Phase 3 trials for Cushing’s syndrome completed |
How will relacorilant be administered in Cushing’s disease?
In Phase 3 clinical trials, people with Cushing’s received relacorilant in the form of oral capsules taken once daily. The starting dose was 100 mg, which was gradually increased to a maximum of 400 mg based on tolerability and clinical responses.
Relacorilant in Cushing’s disease clinical trials
Corcept’s application for relacorilant’s approval was supported by data from two Phase 3 clinical trials involving adults with endogenous Cushing’s who had hypertension and/or hyperglycemia conditions. Those trials were dubbed GRACE (NCT03697109) and GRADIENT (NCT04308590).
- In GRACE, relacorilant was associated with significant reductions in blood pressure, blood sugar, and other Cushing’s symptoms. Compared with individuals who switched to a placebo partway through the trial, those who remained on relacorilant throughout the study were 83% less likely to experience a loss of blood pressure control.
- In GRADIENT, which specifically involved individuals whose Cushing’s was caused by a tumor on the adrenal glands that produces cortisol, relacorilant use led to significant reductions in blood pressure, blood sugar, and body weight compared with a placebo.
Pooled analyses from GRACE and GRADIENT likewise demonstrated the therapy’s beneficial effects on blood pressure, blood sugar, and body composition. Findings from an earlier Phase 2 trial (NCT02804750) also showed similar benefits.
Interim results from an open-label extension study (NCT03604198) involving individuals who completed previous relacorilant studies have suggested that the treatment has a favorable long-term safety and efficacy profile.
Relacorilant side effects
The most common side effects that occurred after relacorilant treatment in clinical trials were:
- pain in the back, extremities, abdomen, joints, or muscles
- headache
- swelling
- nausea
- fatigue
- diarrhea or constipation
- dizziness
- indigestion
- darker spots on the skin
Most side effects were mild or moderate, according to Corcept, and many are consistent with the expected side effects of any treatment for high cortisol levels, the company noted.
Cushing's Disease News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
 Fact-checked by
Fact-checked by 

